Our Research projects
The Enzymology & Metabolism group is involved in research projects focusing on (i) Metabolite/Protein Damage and Repair, (ii) Rare Disease Research and (iii) Metabolomics Approaches applied to Enzyme Discovery and Disease Research.
-
Duration:
-
Funding source:
FNR CORE grant NAXDE (C18/BM/12661133), FNR DTU grant PARKQC (PRIDE17/12244779), Donations from Juniclair foundation and the Lions Club Luxembourg
-
Researchers:
“Adhish Walvekar (Yeast, HAP1 and neuronal/organoid models)
Myrto Patraskaki (myrto.patraskaki@uni.lu) (Zebrafish models)” -
Partners:
John Christodoulou (MCRI, Australia); Jens Schwamborn (LCSB)
-
Description:
In 2011, we discovered a widely conserved enzymatic repair mechanism for hydrated forms of NADH and NADPH, two central cofactors of cell metabolism. The hydration of NAD(P)H can be catalysed as a side reaction of GAPDH (glyceraldehyde 3-phosphate dehydrogenase) or can proceed spontaneously at elevated temperatures, leading to the formation of two epimers of NAD(P)H hydrates designated S-NAD(P)HX and R-NAD(P)HX. Those hydrated derivatives cannot act as enzyme cofactors and have been shown to inhibit dehydrogenases. To cope with these damaged forms of NAD(P)HX, organisms have evolved a repair system that comprises an ATP-dependent S-NAD(P)HX dehydratase (NAXD) and an NAD(P)HX epimerase (NAXE). Mutations in NAXD or NAXE lead to a severe infantile neurometabolic disorder. To elucidate the molecular mechanism of this disorder, we aim in this project to better understand how and under which circumstances NAD(P)HX is formed in the cell and what cellular functions are most affected by the presence of NAD(P)HX. We are therefore investigating the consequences of NAD(P)HX repair deficiency in various models, starting from the simple yeast and human HAP1 cell models to more complex iPSC-derived neuronal cell lines and brain organoids, as well as zebrafish models. In-depth phenotyping of these models is performed at molecular (e.g. metabolomics, transcriptomics) and cellular (e.g. viability, mitochondrial function) levels. The ultimate aim is to pinpoint critical perturbations induced by NAXD and/or NAXE deficiency and to conceive and test strategies to prevent or correct these perturbations. The focus is on small molecule-based approaches with therapeutic potential.
-
Project details (PDF):
-
Duration:
-
Funding source:
-
Researchers:
Leonardo Mastrella; Sebastian Perrone
-
Partners:
Marcelo Guerin (CIC bioGUNE, Spain)
-
Description:
Another recently discovered enzyme potentially involved in metabolite repair is GDP-glucose phosphorylase (GDPGP1). The latter degrades GDP-glucose, a nucleotide sugar that has no known function in mammalian cells and that seems to be erroneously formed by GDP-mannose pyrophosphorylase, the enzyme that normally forms GDP-mannose (GDP-Man). GDP-Man is an important nucleotide sugar used for protein N-glycosylation in the cell. Purification of this enzyme from mammalian tissues revealed that it is composed of two different subunits that are encoded by two distinct genes, GMPPA and GMPPB. While the isolated GMPPB protein has been shown to catalyse GDP-Man formation, the role and function of the GMPPA subunit remains less well understood. In collaboration with Marcelo Guerin’s group at CIC bioGUNE, we currently work on producing, isolating and structurally characterizing the GDPGP1 protein. The relevance of this project is underlined by the fact that defects in both the GMPPA and GMPPB proteins have been found to lead to rare glycosylation disorders. Similarly, GDP-glucose phosphorylase deficiency could be causally involved in glycosylation disorders of currently unknown genetic origin.
-
Project details (PDF):
-
Duration:
-
Funding source:
Uni.lu Internal Research Grant (HYMEPI)
-
Researchers:
Carole Linster
-
Partners:
Lasse Sinkkonen (Department of Life Sciences and Medicine, Uni.lu)
-
Description:
D-2-hydroxyglutarate (D-2HG) and L-2-hydroxyglutarate (L-2HG) are metabolites that accumulate in several types of inherited neurometabolic diseases and certain forms of cancer such as glioblastomas and acute myeloid leukaemias. In each of these diseases, mutations in specific dehydrogenases for L-2HG, D-2HG, or isocitrate have been shown to cause 2HG accumulation. However, the intracellular pathways leading to 2HG formation and the roles of 2HG in disease are still unclear. We recently uncovered the metabolic reactions leading to 2HG formation and degradation in Saccharomyces cerevisiae, an organism that produces exclusively the D-enantiomer of this dicarboxylic acid. Our results show that in yeast, D-2HG metabolism links the main serine synthesis pathway to the mitochondrial respiratory chain. Using an mQTL approach, we search for additional genes involved in controlling D-2HG levels in this model organism. In addition, in collaboration with Lasse Sinkkonen’s group, we investigate the epigenetic effects of 2HG accumulation (and potential consequences thereof) in yeast. This work could lead to the identification of molecular drug target candidates to be tested for the treatment of certain forms of cancer and severe neurometabolic disease.
-
Project details (PDF):
-
Duration:
-
Funding source:
FNR DTU grant PARKQC (PRIDE17/12244779)
-
Researchers:
Chiara Romano
-
Partners:
Rejko Krüger (LCSB)
-
Description:
The Parkinson’s disease associated protein DJ-1 has been proposed to have a deglycase activity towards nucleotides and amino acids modified by the reactive dicarbonyl metabolites, glyoxal and methylglyoxal. Protein and DNA glycation adducts produced by exposure to glyoxal and methylglyoxal were also shown to be repaired in vitro by DJ-1. Glyoxal and methylglyoxal can be formed physiologically by lipid peroxidation and as by-product of glycolysis, respectively. Very recently, DJ-1 was also shown to act on a reactive metabolite derived from the glycolytic intermediate 1,3-bisphosphoglycerate. We generated yeast and mammalian cell models deficient in DJ-1 function to investigate metabolic consequences of this deficiency using mass-spectrometry-based metabolomic approaches.
-
Project details (PDF):
-
Duration:
-
Funding source:
EU H2020 grant SinFonia (GA 814418)
-
Researchers:
Corey Griffith
-
Partners:
Pablo Ivan Nikel (DTU, Denmark)
-
Description:
“SinFonia aims to integrate the nonnative element fluorine into the metabolism of Pseudomonas putida to produce novel fluorinated polyhydroxyalkanoates (PHAs), ideally in such a way that bacterial growth will become dependent on this incorporation. Fluorine is common in industrial chemicals with versatile applications from electrical insulation to waterproofing, yet it is seldom present in biological systems. The current production processes for fluorochemicals often negatively affect the environment, and there is a great need for more sustainable and less harmful alternatives. If successful, SinFonia will provide a less hazardous and more sustainable solution to synthesizing fluorochemicals.
Our role, in collaboration with the Environmental Cheminformatics Group, is to identify fluorinated and undesirable metabolites in engineered bacteria using non-targeted mass spectrometry. Engineered systems may lack sufficient metabolite repair capacity, which we aim to counteract by screening for metabolite damages in our cell factories and envisaging strategies to repair them. Metabolite repair may be particularly important in SinFonia due to the load of adding heterologous pathways and a nonnative element to create new-to-nature products “ -
Project details (PDF):
-
Duration:
-
Funding source:
Donations from ATOZ foundation and private sponsors, LCSB Internal Flagship Grant
-
Researchers:
Ursula Heins-Marroquin; Ana-Maria Osiceanu
-
Partners:
Anne Grünewald (LCSB); Jens Schwamborn (OrganoTherapeutics)
-
Description:
Mutations in ATP13A2 (also designated PARK9) were described in 2006 to cause the Kufor-Rakeb Syndrome, a rare early-onset form of Parkinsonism with dementia. Interestingly, in 2012, a different mutation in the same gene was found to lead to neuronal ceroid lipofuscinosis (also known as Batten disease) and in 2017, yet other ATP13A2 mutations were associated with Spastic Paraplegia-78. The link between these rare neurodegenerative diseases is further supported by studies in animal models, but the molecular mechanism(s) underlying the connection between ATP13A2 deficiency and disease pathogenesis is not yet understood. Nevertheless, these findings indicate that ATP13A2 plays a crucial role in supporting healthy neuronal cells. In this project, we developed a drug screening pipeline to accelerate the discovery of potential therapeutic compounds for ATP13A2 associated disorders. We took advantage of the fact that ATP13A2 is highly conserved from yeast to humans and we developed a high-throughput drug screening strategy for ATP13A2 deficiencies in yeast based on a decreased zinc resistance phenotype. We have applied it to implement a primary screen of 2560 drugs. For validation of the positive hits, we generated two stable ATP13A2 knockout zebrafish lines that showed increased sensitivity to manganese toxicity, especially in the central nervous system. Based on phenotypic rescue assays, we validated 2 hits from the primary yeast screen, N-acetylcysteine and furaltadone, in zebrafish. Currently we are testing these two drugs in ATP13A2 patient-derived cells. We aim in the future to keep exploiting our unique combination of ATP13A2 models for further investigation of related disease mechanisms as well as small molecule-based therapeutic approaches.
-
Project details (PDF):
-
Duration:
-
Funding source:
FNR bilateral AFR postdoc grant (17156445), Donations from the Losch foundation, Quilvest, the Rotary Club Luxembourg and private sponsors
-
Researchers:
Ursula Heins-Marroquin
-
Partners:
Nancy Braverman (McGill University)
-
Description:
“Zellweger syndrome (ZS) is a rare peroxisomal biogenesis disorder. It affects several organs (including brain, liver and kidneys) and symptoms typically appear in the newborn period. Mutations in the PEX1 gene are the most common cause for ZS. There is no cure for ZS and good disease models are rare. Our project is focused on development of a robust high-throughput drug screening approach in PEX1-deficient ZS patient-derived cell lines as well as the development of a pex1-deficient vertebrate model. The overall aim is to discover compounds that can alleviate the underlying cause of ZS, namely peroxisomal dysfunction, by first screening thousands of compounds in patient-derived cells and potentially advancing to a whole organism model for validation, in our case zebrafish (Danio rerio).
In the human cell line screens, our readout is subcellular localization of catalase via immunostaining and fluorescence imaging. We collaborate with the LCSB/LIH Disease Modelling and Screening platform to perform the imaging-based drug screens in 384-well plate format followed by automated data analysis using MATLAB scripts. The pex1 mutant zebrafish model is being generated using CRISPR/Cas9 technology. This is a very exploratory part of the project and phenotypes in this model will be analysed carefully in collaboration with Nancy Braverman’s group at McGill University. Ultimately, we aim to validate positive hits from the cell-based screens in zebrafish through potential phenotypic rescue assays.” -
Project details (PDF):
-
Duration:
-
Funding source:
FNR ATTRACT fellowship to Emma Schymanski ECHIDNA (A18/BM/12341006)
-
Researchers:
Lorenzo Favilli (co-supervised PhD student from Environmental Cheminformatics Group)
-
Partners:
Emma Schymanski (LCSB)
-
Description:
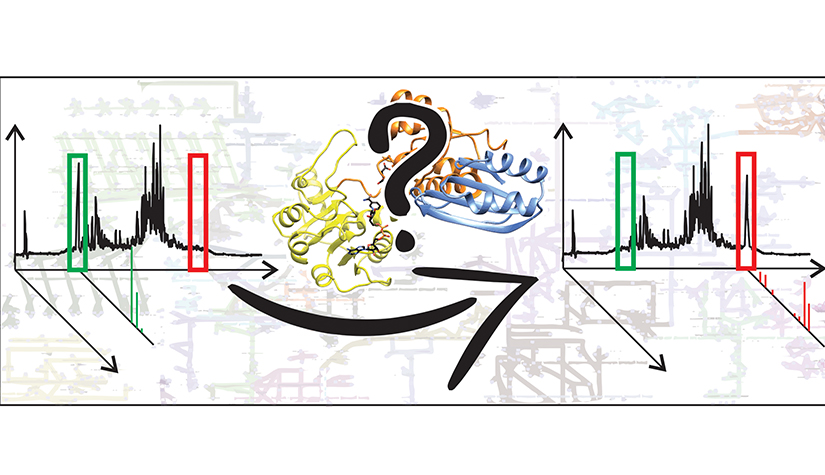
In collaboration with the Bioinformatics Core, we identified more than 600 and 2000 putative enzyme genes among the about 2000 and 6500 genes of unknown function in yeast and human, respectively. Prioritising putative enzyme genes with predicted roles in metabolism and/or disease, we develop, in collaboration with the Metabolomics platform hosted by our group and with the Environmental Cheminformatics Group, targeted and non-targeted LC-MS-based approaches to compare metabolite profiles of control cells and cells with specific gene deletions. Metabolites whose levels differ significantly between these cells help to predict endogenous reactions catalysed by the enzymes of interest. Those predictions can then be validated on recombinant protein level through appropriate enzymatic assays. This approach has already allowed to identify a new eukaryotic D-ribulokinase. With this project, we aim to address a major post-genomic challenge that can only be tackled by a community-wide effort: progress from knowing the code to understanding the message by decreasing the number of genes of unknown function.
-
Project details (PDF):
-
Duration:
-
Funding source:
FNR DTU grant MICROH (PRIDE17/11823097); PMC 2019 grant MetPM
-
Researchers:
Marat Kasakin
-
Partners:
Guido Bommer (de Duve Institute, Belgium); Elisabeth Letellier (Department of Life Sciences and Medicine, UL)
-
Description:
“Hydrogen sulfide (H2S) and persulfides have been shown to affect signalling pathways and metabolic functions involved in carcinogenesis. H2S is produced in mammalian cells through the transsulfuration pathway, but intestinal cells are also exposed to H2S produced by commensal gut bacteria. The sulfur oxidation pathway, involving the SQRDL and ETHE1 enzymes, plays a key role in catabolism of H2S in mammalian cells.
The aims of the project comprise (1) elucidation of the mammalian sulfur oxidation pathway and the potential role of alterations in this pathway in colorectal cancer, (2) investigation of metabolomic profile changes, and more specifically in sulfur metabolism, in colorectal cancer development and progression, and (3) analysis of the influence of the gut microbiome on sulfur metabolism in the host intestinal epithelium. Gaining a deeper insight into the molecular mechanisms of the microbiome-host crosstalk is essential for the development of dietary recommendations, notably in the context of colorectal cancer.” -
Project details (PDF):
-
Duration:
-
Funding source:
FNR POC grant HAPPY (PoC19/13593527)
-
Researchers:
Ursula Heins-Marroquin
-
Partners:
Nicole Paczia (MPI Marburg); Pranjul Shah (University of Luxembourg)
-
Description:
Our group has built up a platform for using yeast as a model organism, notably for functional genomics purposes. Yeast is the simplest eukaryotic organism, but, due to its ease of manipulation and amenability to genetic modifications, has proven invaluable in the elucidation of conserved molecular mechanisms underlying important cell functions such as mitochondrial biogenesis, cell division, and cell death. Many human disease genes have counterparts in yeast, which can therefore also be used for the dissection of molecular mechanisms of disease and for drug screens. Gene deletion collections in Saccharomyces cerevisiae for all non-essential genes are hosted by the platform as well as a collection of natural yeast strains and humanized yeast models (alpha-synuclein overexpression strains). Production and subsequent purification of recombinant proteins of interest by overexpression in yeast can be performed. Yeast-specific metabolite and RNA extraction protocols for subsequent metabolomics and transcriptomics analyses, and high-throughput phenotypic screens and lifespan assays have been developed to allow detailed functional analyses of genes of interest and to assist metabolic modelling efforts. Currently, a new design for microfluidics-based determination of yeast replicative lifespan is being tested
-
Project details (PDF):