The FastCoV project has two main phases. The first is a pilot phase with a small group of 20 participants to validate the study design and feasibility. After evaluation of the pilot phase, the main clinical trial will start with more participants and possible adjustments to the study protocol based on the results of the pilot study.
In the pilot phase, 20 participants with long COVID syndrome will be recruited from the CHNP Rehaklinik. They will undergo a fasting intervention using the Buchinger-Wilhelmi method (see below). Biomedical samples (blood, saliva, urine and stool) will be taken at various times before, during and after the fasting period, and participants will complete questionnaires about their symptoms and well-being using a smartphone app. The aim of this phase is to assess the feasibility of this fasting intervention protocol.
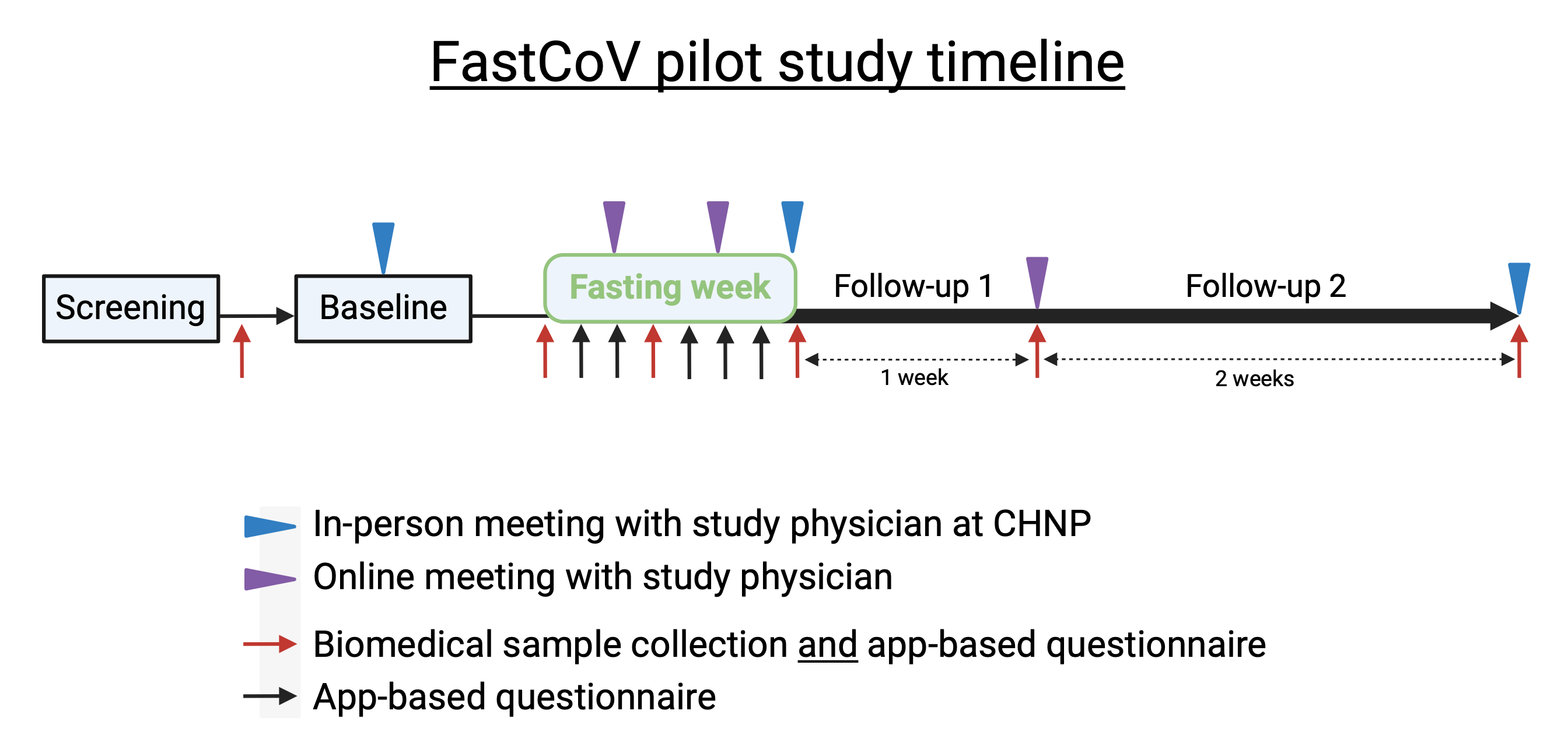
After enrolment, participants will undergo an initial collection of biomedical samples (blood, stool, urine & saliva). Shortly after this, they will have a face-to-face meeting with the study physician where all procedures will be explained in detail and any questions will be answered. They will also complete a smartphone app-based questionnaire for the first time.
Three days before the start of the fast, participants should start to avoid stimulants such as coffee, alcohol and nicotine, and gradually reduce their food intake to carbohydrates only.
On the first day of the fast, participants take a laxative to help empty their bowels, similar to preparing for a colonoscopy. The fast lasts seven days, during which participants consume up to 350 kcal per day from vegetable juices and broth, which are provided by the study team.
During the fasting week, participants will complete the app-based questionnaire every day. Biomedical samples will be taken at the beginning, end, and once during the week, and an online meeting with the study physician will occur twice. Participants will meet with the study physician on the last day of fasting.
Participants will break the fast with an apple and then gradually return to a whole-food diet. This will be done according to the guidelines of the German Nutrition Society, which will be explained to participants during the face-to-face meeting with the study physician on the last day of fasting.
Participants will be followed for three weeks after the fast. After the first week, biomedical samples will be collected and participants will meet online with the study physician. Two weeks later, biomedical samples will be collected one last time and participants will meet with the study physician in person.
Following the pilot phase, the main clinical trial will recruit 64 additional participants with long COVID syndrome at the CHNP Rehaklinik. Participants will undergo the same or a similar fasting intervention, sample collection, and questionnaire-based assessment as in the pilot study, subject to any protocol adjustments based on the pilot study’s results.
For the main clinical trial, participants will be randomly assigned to two groups: one group will start fasting immediately and the other group will wait for two weeks during which time they will eat their usual diet. After the fasting week, half of the participants in each group will follow an anti-inflammatory diet, while the other half will return to their usual diet. Participants in the main clinical trial will be followed for one year, with samples and a questionnaire-based assessment taken every three months.
The main objective is to determine whether the fasting intervention improves the symptoms of long COVID syndrome and whether an anti-inflammatory diet maintains these benefits longer than a normal diet.
The fasting protocol

Recruitment
Participants in the FastCoV study (pilot phase and main clinical trial) are recruited through the long COVID treatment division of the CHNP Rehaklinik. They must have been diagnosed with long COVID syndrome, be between 18 and 79 years old, understand the participant information and give written consent.
Key exclusion criteria:
- Contraindication for fasting regime (i.e. underweight, recent weight loss, intestinal or digestive disease).
- Existing vegan or calorie-restricted diet in the last six months.
- Antibiotic use during the last 12 months.
- Pregnancy or breastfeeding.
- Use of anti-psychotic drugs
For more information on recruitment and the complete list of inclusion and exclusion criteria, please contact the study team.
Support and monitoring
The dieticians and medical team at CHNP will be with the participants throughout the study, providing detailed instructions and support. The team at the Charité University Hospital in Berlin will act as external consultants to help analyse and interpret the results. Adherence to the fasting protocol will be monitored using a digital application (MyCap) and urine ketone measurements.