Shortly after the start of the COVID-19 pandemic in 2020, it became clear that some people experienced lingering effects beyond the acute phase of the initial SARS-CoV-2 infection. This condition is now known as long COVID syndrome and refers to a range of symptoms that persist for weeks or months after the initial infection has resolved. Common symptoms include fatigue, shortness of breath, brain fog, joint pain and chest pain.
Below you will find more information about long COVID syndrome, its impact on health and society, current treatment approaches and the FastCoV study team’s previous experience in nutritional clinical trials.
Long COVID syndrome is a highly variable condition that occurs as a post-acute complication of SARS-CoV-2 infection in up to 10% of cases. According to the World Health Organization, long COVID syndrome is defined as symptoms that occur at least 3 months after initial infection, persist for at least 2 months, and cannot be attributed to an alternative diagnosis.
The duration, characteristics and severity of symptoms are highly variable, ranging from fatigue, shortness of breath, pain, depression/anxiety to cognitive impairment, often referred to as ‘brain fog’. They often affect daily activities, income and quality of life of those affected. The underlying causes of long COVID syndrome are still under investigation.
Current hypotheses focus on:
- Dysfunction of the inflammatory/immune response
- Blood vessel damage
- Cellular metabolic dysregulation
- Disruption to the digestive system and its microbiome.
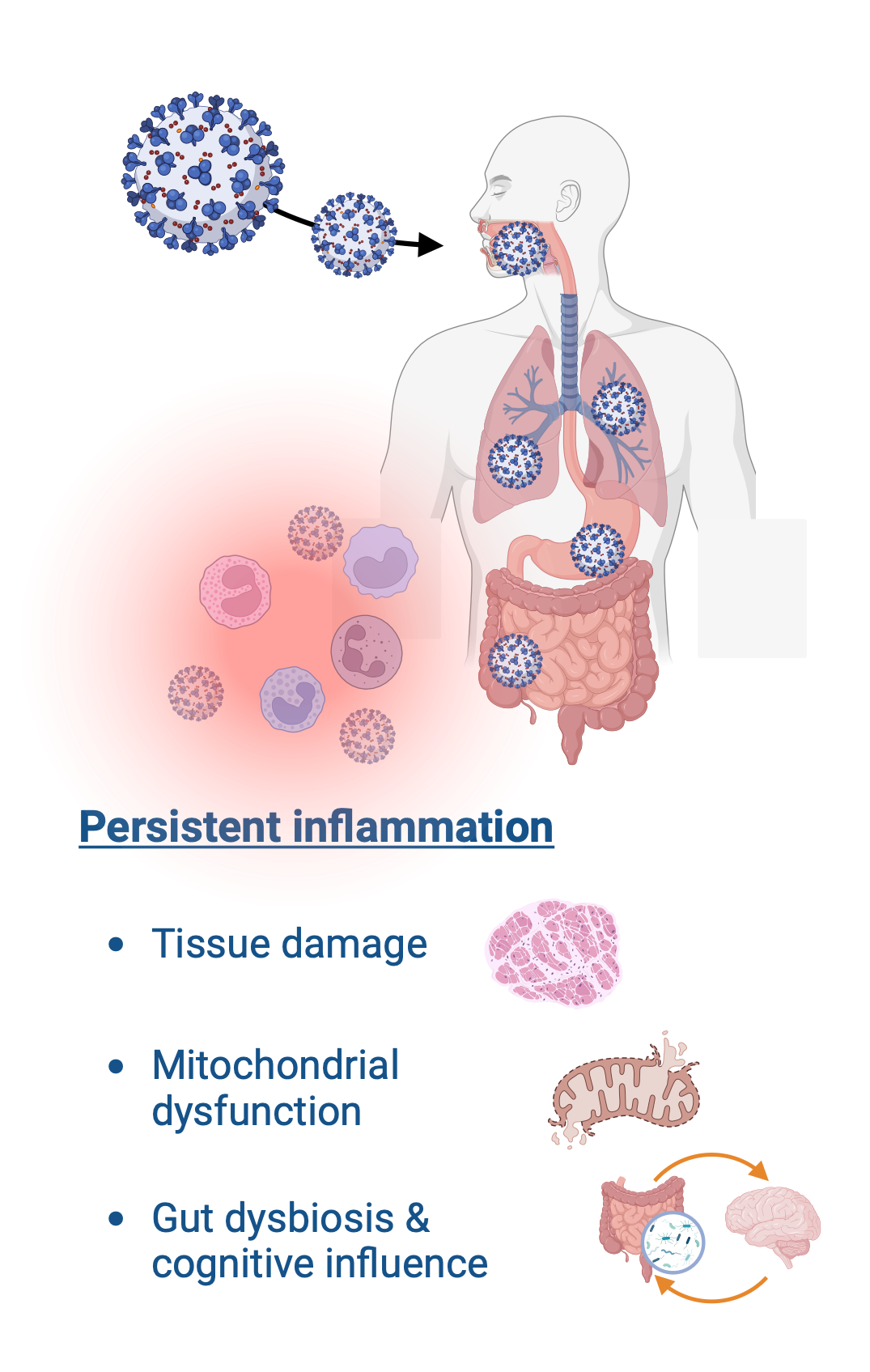
It is thought that the initial infection triggers an immune response that causes inflammation as part of the normal defense mechanism. In some cases, an exaggerated or dysregulated immune response results in persistent inflammation and organ damage. This process is common to other chronic diseases such as rheumatoid arthritis, diabetes and neurodegenerative diseases.
More than 65 million people worldwide are currently affected by various forms of long COVID syndrome, with most patients between 36 and 50 years of age. Most cases involve non-hospitalised individuals who experience symptoms that disrupt their health and quality of life. In addition, long COVID syndrome has a social impact, particularly in the workplace, as many patients have to reduce or stop working due to the condition. This has socio-economic consequences, including increased sickness absence, reduced productivity, social isolation and increased emotional distress.
Although the development of pharmacotherapies such as anti-inflammatory, antiviral, and immunomodulatory treatments appears promising, their efficacy in the treatment of long COVID syndrome has not yet been demonstrated and alternative therapeutic solutions are thus urgently needed. Although traditional pharmacotherapies may alleviate acute symptoms, they are often inadequate to address the complexity of diverse symptoms involving multiple organ systems with unclear underlying mechanisms and increased variability between individuals. To date, patient management involves personalised care plans tailored to each individual’s symptoms to improve quality of life. However, these symptomatic treatments usually do not target the underlying cause, highlighting the importance of research and development in this area.
Recent research suggests that calorie restriction and fasting diets may reduce the inflammatory processes, one of the potential underlying causes of long COVID syndrome and medically supervised fasting has shown beneficial effects in other inflammatory syndromes. In addition, periodic fasting, where people regularly alternate between normal eating and fasting, promotes healthy microbial diversity in the gut. For example, one study showed that 13 out of 14 patients with chronic inflammatory bowel disease who fasted under medical supervision experienced an improvement in their symptoms.
Therefore, we believe that the complex interplay between the immune response, metabolism, and gut microbiome in long COVID syndrome may be modulated by fasting. However, the lack of standard fasting protocols and limited objective assessment of protocol adherence by study participants pose difficulties in implementing such interventions and hamper the validity and reproducibility of their conclusions. This project aims to overcome these shortcomings and includes a pilot phase to assess feasibility.
The FastCov study team consists of researchers from different Luxembourgish and German institutions. Each group has extensive expertise in studying the impact of diet on various aspects of health and disease.
The group at the Luxembourg Centre for Systems Biomedicine of the University of Luxembourg has recently studied the impact of diet on cognitive function in elderly people with age-related brain pathologies, analysing whether specific dietary interventions could offer neuronal protection. In addition, together with their colleagues from the Department of Life Sciences and Medicine, they have developed the protocol for the ExpoBiome study, a multi-centre clinical trial focusing on rheumatoid arthritis and Parkinson’s disease patients in Berlin and Kassel, Germany. This study investigates the relationship between the gut microbiome, fasting, and immune status.
The team at the Charité hospital in Berlin contributed to the NutriFast study, which investigated the efficacy of therapeutic fasting and a plant-based diet in people with rheumatoid arthritis. Using dried blood spot metabolomics, the researchers were able to identify several metabolites that are upregulated during fasting exercise, indicating specific pathways involved in response to calorie restriction.
Participation in the FastCoV study therefore puts you in the hands of an experienced team, who will guide you throughout the study, from an initial information session, through regular appointments during the fasting phase, to the gradual return to a normal diet and the follow-up.
1. A. V. Raveendran, R. Jayadevan, and S. Sashidharan, “Long COVID: An overview,” Diabetes Metab. Syndr., vol. 15, no. 3, pp. 869–875, 2021, doi: 10.1016/j.dsx.2021.04.007.
2. D. L. Sykes, L. Holdsworth, N. Jawad, P. Gunasekera, A. H. Morice, and M. G. Crooks, “Post-COVID-19 Symptom Burden: What is Long-COVID and How Should We Manage It?,” Lung, vol. 199, no. 2, pp. 113–119, Apr. 2021, doi: 10.1007/s00408-021-00423-z.
3. L. Kohn et al., “Long COVID and return to work: a qualitative study,” Occup. Med. Oxf. Engl., vol. 74, no. 1, pp. 29–36, Feb. 2024, doi: 10.1093/occmed/kqac119.
4. M. S. Durstenfeld et al., “Association of nirmatrelvir for acute SARS-CoV-2 infection with subsequent Long COVID symptoms in an observational cohort study,” J. Med. Virol., vol. 96, no. 1, p. e29333, 2024, doi: 10.1002/jmv.29333.
5. X. Y. Loke, S. A. M. Imran, G. J. Tye, W. S. Wan Kamarul Zaman, and F. Nordin, “Immunomodulation and Regenerative Capacity of MSCs for Long-COVID,” Int. J. Mol. Sci., vol. 22, no. 22, Art. no. 22, Jan. 2021, doi: 10.3390/ijms222212421.
6. N. Nagata et al., “Human Gut Microbiota and Its Metabolites Impact Immune Responses in COVID-19 and Its Complications,” Gastroenterology, vol. 164, no. 2, pp. 272–288, Feb. 2023, doi: 10.1053/j.gastro.2022.09.024.
7. F. Grundler, R. Mesnage, A. Cerrada, and F. Wilhelmi de Toledo, “Improvements during long-term fasting in patients with long COVID – a case series and literature review,” Front. Nutr., vol. 10, p. 1195270, Nov. 2023, doi: 10.3389/fnut.2023.1195270.
8. B. Hansen, K. Roomp, H. Ebid and J.-G. Schneider. “Perspective: The Impact of Fasting and Caloric Restriction on Neurodegenerative Diseases in Human,” Adv Nutr. 2024 Apr;15(4):100197. doi: 10.1016/j.advnut.2024.100197.
9. B. Hansen et al., “Protocol for a multicentre cross-sectional, longitudinal ambulatory clinical trial in rheumatoid arthritis and Parkinson’s disease patients analysing the relation between the gut microbiome, fasting and immune status in Germany (ExpoBiome),” BMJ Open, vol. 13, no. 8, p. e071380, Aug. 2023, doi: 10.1136/bmjopen-2022-071380.
10. A. M. Hartmann et al., “Post Hoc Analysis of a Randomized Controlled Trial on Fasting and Plant-Based Diet in Rheumatoid Arthritis (NutriFast): Nutritional Supply and Impact on Dietary Behavior,” Nutrients, vol. 15, no. 4, Art. no. 4, Jan. 2023, doi: 10.3390/nu15040851.