Parkinson’s disease is a progressive neurodegenerative disease, but how it spreads through the brain remains poorly understood. Now, researchers from the Luxembourg Centre for Systems Biomedicine (LCSB) and international collaborators have successfully demonstrated the progressive spread of alpha-synuclein aggregation in a patient-derived assembloid model. Understanding how toxic protein aggregates move between brain regions is crucial to unravelling the mechanisms of early disease progression. The study, published in Advanced Science, provides new insights into this process.
Investigating the spread of protein aggregates
While Parkinson’s disease is often associated with symptoms like tremors, stiffness, and slowed movement, the disease actually begins silently, long before these motor symptoms appear. The presence of early, non-motor symptoms has led researchers to investigate how Parkinson’s spreads through the nervous system over time. One leading theory, first proposed by German anatomist Prof. Heiko Braak, suggests that Parkinson’s disease may start in the gut or lower brainstem, before gradually moving upward through the brain in a specific pattern. However, the precise mechanisms driving this spread have remained elusive.
At the centre of the disease is a protein called alpha-synuclein. In healthy brains, it helps to regulate communication between neurons. In Parkinson’s, this protein misfolds and aggregates, damaging nerve cells and forming abnormal structures known as Lewy bodies – a hallmark of the disease. Understanding how these aggregates propagate between brain regions is critical for developing new treatment strategies. To tackle this challenge, the Developmental & Cellular Biology group at the LCSB, led by Prof. Jens Schwamborn, has collaborated with researchers from Eindhoven University of Technology, Universitätsklinikum Schleswig-Holstein in Lübeck, and the Hellenic Pasteur Institute in Athens to recreate the early stages of Parkinson’s disease in the lab using an innovative tool: Brain assembloids.
Brain assembloids: A novel model for disease research
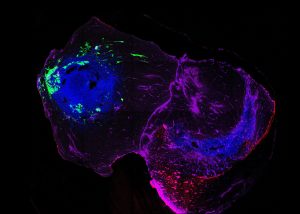
To investigate Parkinson’s disease progression, the researchers developed assembloids fused from two types of brain organoids. These are miniature, lab-grown brain structures made from human stem cells. One organoid represented the hindbrain, where Parkinson’s disease is thought to begin, and another one represented the midbrain, home to the dopamine-producing neurons that are destroyed in the disease.
Using induced pluripotent stem cells (iPSCs) derived from Parkinson’s patients with a rare SNCA gene triplication, a mutation that causes an overproduction of alpha-synuclein, the team found that hindbrain organoids spontaneously developed toxic protein aggregates. When these were then connected with healthy midbrain organoids to form an assembloid, the alpha-synuclein aggregates began spreading into the healthy midbrain tissue within just 30 days.
“This patient-derived assembloid model gives us an unprecedented look at how toxic alpha-synuclein accumulates and spreads between brain regions,” said Prof. Jens Schwamborn. “It is the first time we have been able to observe this pathology progression in a human-based system without overexpressing proteins or relying on artificial triggers.” The study hence provides direct evidence for Braak’s hypothesis, demonstrating that toxic alpha-synuclein aggregates can propagate from one brain region to another.
Alpha-synuclein and its role in Parkinson’s disease progression
The study showed that the presence of excess alpha-synuclein in the hindbrain was sufficient to trigger its spread to the midbrain, impacting synapses – the vital communication points between neurons. Even before neurons began to die, researchers observed early signs of synaptic dysfunction, a key step in of neurodegeneration.
“An increase in alpha-synuclein in the hindbrain alone was sufficient to initiate the spread of pathological alpha-synuclein protein to the healthy midbrain, leading to synaptic dysfunction,” explained Prof. Schwamborn. “This qualifies the model for future applications to both gain a deeper understanding of the pathology spreading process and develop therapeutic approaches inhibiting this process.“
The team also used advanced techniques like microelectrode arrays and viral tracing to confirm that the organoids formed functional, directional connections, just like real brain regions. As alpha-synuclein spread, these connections weakened, indicating early disruption of brain network activity.
Advancing Parkinson’s research: Toward early detection and personalized medicine
Assembloids provide a controlled environment to study Parkinson’s progression with unprecedented detail. By mimicking the brain’s natural architecture and connectivity, they hold great promise not only for uncovering the earliest events in Parkinson’s disease but also for finding new approaches for precision medicine. As the organoids are derived from individual patients, researchers can study how specific genetic backgrounds affect disease development, and test targeted treatments.
“This model offers a unique opportunity to study early disease mechanisms and screen for drugs that could block the spread of alpha-synuclein before irreversible damage occurs,” said Prof. Schwamborn. “Ultimately, this could lead to preventive therapies for those at high genetic risk, stopping the disease before it spreads.”
—
Scientific publication: Gomez-Giro G, Frangenberg D, Vega D, Zagare A, Barmpa K, Antony PMA, Robertson G, Sabahi-Kaviani R, Haendler K, Kruse N, Papastefanaki F, Matsas R, Spielman M, Luttge R and Schwamborn JC. Alpha-synuclein pathology spreads in a midbrain-hindbrain assembloid model. Advanced Science, April 2025.
Funding: This research was supported by the European Union’s Horizon 2020 Research and Innovation Programme.
Top image credit: Gemma Gomez-Giro.