Researchers of the Luxembourg Centre for Systems Biomedicine (LCSB) keep unfolding the molecular mechanisms underlying neurodegeneration in Parkinson’s disease. In a collaborative effort bringing together different teams, they highlight the impact of a mutation affecting the Miro1 protein. With its link to altered energy metabolism, alpha-synuclein accumulation and loss of dopaminergic neurons, this protein confirms the significant effect of some rare genetic variants and the relevance of mitochondrial pathologies in Parkinson’s disease.
A newcomer in Parkinson’s genetic landscape
Complex by nature, Parkinson’s disease is not only clinically but also a genetically heterogenous. If a few well-known mutations are associated with the disease, there is growing evidence that rare variants in different genes might also have substantial effects. RHOT1 has been identified as one of the genes with the highest burden of rare genetic variants in people with Parkinson’s, meaning alterations in this gene could play an important role in genetic susceptibility to the disease.
Miro1, a protein encoded by RHOT1, has recently been linked to the disease. It is essential for maintaining equilibrium in mitochondria, key components of the human cells, and it interacts with other proteins that are key players in Parkinson’s. In a new study, just published in Brain, LCSB researchers investigated a specific mutation in Miro1 and the role it plays in different cellular activities and pathways relevant for neurodegeneration.
“After describing the first patients carrying distinct mutations in the RHOT1 gene encoding Miro1 several years ago, we wanted to validate the underlying pathological mechanisms using advanced gene-editing technologies in vitro and in vivo,” explains Prof. Rejko Krüger, head of the Translational Neuroscience group at the LCSB and co-author of the study.
Combining models for a better overview
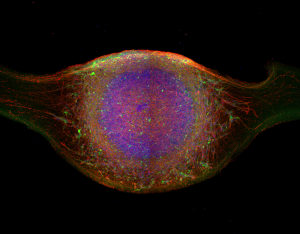
The scientists performed experiments in patient-derived models in vitro and in genetically engineered in vivo models. They first used two types of cell cultures derived from pluripotent stem cells, originating either from a patient carrying the Miro1 mutation or healthy controls: Midbrain organoids, 3D cell cultures that mimic a specific region of the human brain, and cultures of dopaminergic neurones.
“These patient specific cell culture models allow us to investigate the effects of Miro1 mutations at the cellular level. Particularly brain organoids recapitulate the complexity of the human midbrain and thereby make it possible to study what happens when the Miro1 protein is mutated not only in a single cell but in the complex interaction of various cell types, including dopaminergic neurons and astrocytes,” details Prof. Jens Schwamborn, principal investigator of the Developmental & Cellular Biology group.
Additionally, to study the effect of the mutation in vivo, the researchers developed the first Miro1-mutant mouse model. By introducing a mutation homologous to the one existing in humans with CRISPR/Cas9 technology, they could observe changes typical of Parkinson’s disease in the brain as well as in the behaviour of mice over time.
Connecting the dots: Mitochondria, calcium signalling and alpha-synuclein
Taken together, the results obtained in the organoids, the neuronal cultures and the mice showed that the Miro1 mutation is sufficient to cause the loss of dopaminergic neurons, likely mediated through mitochondrial dysfunction. “There is increasing evidence that mitochondrial impairment is a major driver of neurodegeneration. The results from the current study further strengthen this finding: The mutation in Miro1 seems to increase susceptibility to mitochondrial damage, which impacts the energy production and ultimately leads to cell death in the investigated models,” underlines Prof. Anne Grünewald, head of the Molecular & Functional Neurobiology group.
The study goes further, unveiling a novel molecular mechanism linking Miro1 and α-synuclein accumulation, a hallmark of Parkinson’s disease. The mutation disrupts the regulation of calcium inside the cells, leading to the activation of a protein that cleaves α-synuclein and promoting its aggregation. This accumulation may in turn exacerbate mutation-dependent mitochondrial damage, resulting in the loss of dopaminergic neurons.
Collaborative work brings significant results
Building on the interdisciplinary skills of different teams at the LCSB, together with international partners, has made these results possible. Three LCSB research groups brought their expertise to the table, from the organoid methodology developed by the Developmental & Cellular Biology group to the mitochondria know-how of the Molecular & Functional Neurobiology group and the deep understanding of the genetic basis of Parkinson’s and its validation in patient-derived models of the Translational Neuroscience group. Technical knowledge also played a key part with the contributions of the rodent facility and Bioimaging Platform for the experimental work with mouse models and image-based analyses.
Pulling these resources together, a strategy at the core of the LCSB, led to significant findings with numerous implications. The study further demonstrates the importance of genetic variance in Parkinson’s disease susceptibility. It provides a new mouse model of Parkinson’s that comprehensively encompass key characteristics of the disease and it identifies Miro1 as a convergent player in neurodegeneration. This protein is now a potential drug target and molecular biomarker, relevant for modelling Parkinson’s disease and developing new disease-modifying therapies.
—
Scientific publication: Axel Chemla, Giuseppe Arena, Ginevra Sacripanti, Kyriaki Barmpa, Alise Zagare, Pierre Garcia, Vyron Gorgogietas, Paul Antony, Jochen Ohnmacht, Alexandre Baron, Jaqueline Jung, Frida Lind-Holm Mogensen, Alessandro Michelucci, Anne-Marie Marzesco, Manuel Buttini, Thorsten Schmidt, Anne Grünewald, Jens Schwamborn, Rejko Krüger & Cláudia Saraiva, Parkinson’s disease mutant Miro1 causes mitochondrial dysfunction and dopaminergic neuron loss, Brain, February 2025.
Top picture by Dr Cláudia Saraiva