In a recent study, two teams from the Luxembourg Centre for Systems Biomedicine (LCSB) of the University of Luxembourg, as well as national and international partners, collaborated to explore the role of diet and the gut microbiome in the progression of Parkinson’s disease. Their results, published in the scientific journal Cell Reports, show that dietary fibre deprivation and exposure to a specific bacterial protein can exacerbate the disease, highlighting the importance of lifestyle management for people with Parkinson’s disease.
Parkinson’s disease is a neurological disorder characterised by the loss of specific neurons in the brain and the aggregation of a human protein called α-synuclein. While several risk factors for this disease are well-known, it is not yet clear what can modulate its progression over time. In this context, researchers from the Systems Ecology group and the Neuropathology group at the LCSB combined their respective expertise to study the effects of diet, the gut microbiome’s composition and proteins secreted by these microorganisms in a mouse model of Parkinson’s disease.
“Based on a series of tests, we determined that a specific transgenic mouse line, that replicates key features of Parkinson’s disease, was an appropriate model to investigate the impact of environmental factors, such as diet, on the evolution of this disease,” explains Dr Kristopher Schmit, who conducted the study during his PhD within the Systems Ecology and the Neuropathology groups. “These mice overexpress the protein α-synuclein which leads to the progressive apparition of motor symptoms, just like in patients.”
When these transgenic mice were fed a diet with low amounts of fibre, the microbial diversity in their gut decreased. In particular, bacteria associated with neuroprotective functions declined, whereas mucus-foraging ones increased, leading to a thinning of the outer mucus layer in the colon. “As the mucus layers act as a physical barrier against pathogens, mucus erosion driven by changes in the microbiome could facilitate access of pathogenic agents to the deeper layers of the gut, notably reaching its nerve cells,” details Prof. Paul Wilmes, head of the Systems Ecology group.
Additionally, when combining fibre deprivation with the exposure to curli, a protein produced by bacteria such as Escherichia coli and which can accelerate the aggregation of human α-synuclein, the gut barrier integrity was further disrupted. The presence of curli also led to an increase in α-synuclein aggregation in the peripheral nerves populating the colon. “Surprisingly, on top of affecting the gut itself, we then saw that this process propagated to the brain,” continues Prof. Wilmes.
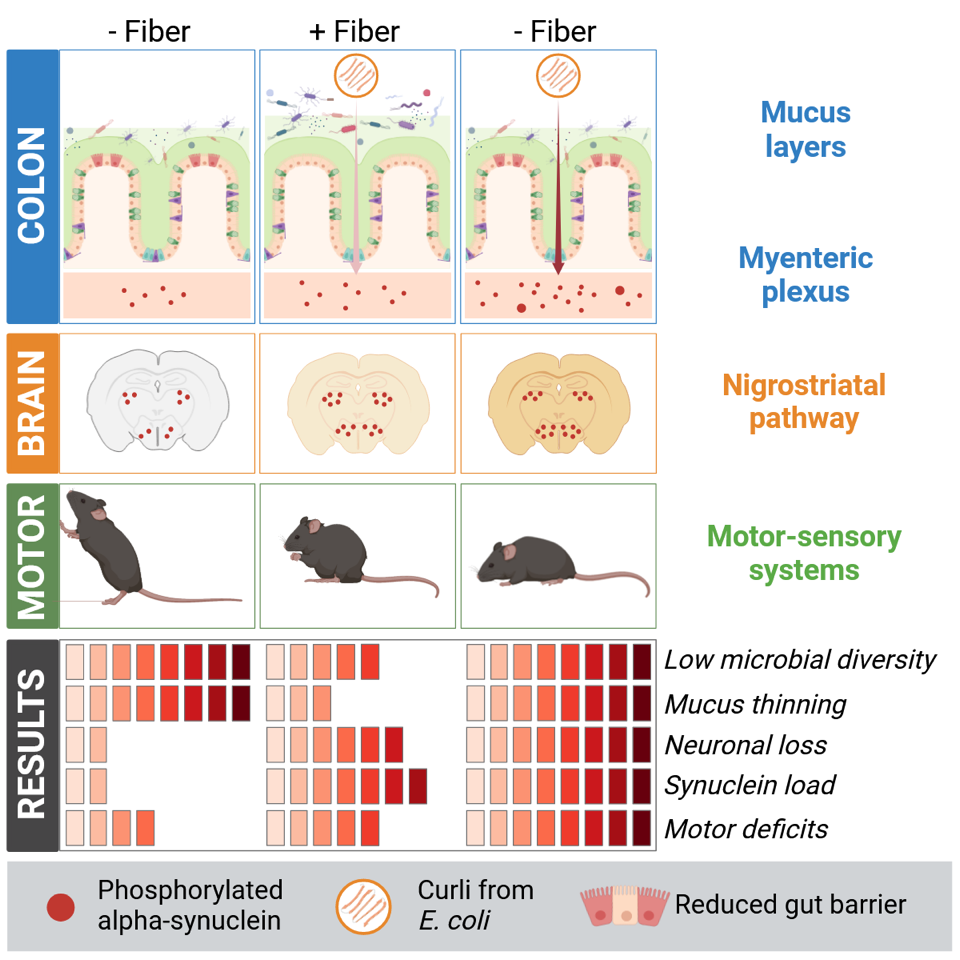
When the researchers from the Neuropathology group explored the effect of this combinatory treatment on the motor behaviour and neuropathologies of the transgenic mice, they first observed that it exacerbated motor deficits, with the mice exposed to curli and low fibre showing reduced coordinative ability over time. Similarly, α-synuclein aggregation in the brain intensified with the presence of curli in the gut and increased further with fibre deprivation. “Moreover, when assessing the loss of neurons in the brain of these mice, we concluded that curli combined with a low amount of fibre drove neurodegeneration in this model of Parkinson’s disease,” explains Dr Manuel Buttini, senior researcher in the Neuropathology group led by Prof. Michel Mittelbronn.
Taken together, these results show that, to different extents, diet and exposure to bacterial proteins produced by the microbiome worsen motor performance as well as intestinal and brain pathologies. The authors outline the possible mechanism at play: “To explain our findings, we propose the following sequence of events, whereby chronic dietary fibre deprivation leads to changes in microbial populations, also called dysbiosis. This then sparks gut mucus erosion and increased gut permeability, facilitating entry of pathogenic agents. In particular, the resulting higher exposure of enteric neurons to proteins such as curli causes greater α-synuclein aggregation in the enteric and central nervous system, leading to neurodegeneration and exacerbation of motor deficits.”
The study sheds important insights on how an environmental factor such as diet and its action through the gut microbiome can modulate Parkinson’s disease progression via the gut-brain axis. It also has implications for lifestyle adjustments that could mitigate the evolution of the disease, such as ensuring an optimal balanced diet and oversight of the use of antibiotics that could favour the proliferation of harmful bacteria like those expressing curli.
—
Reference: Kristopher J. Schmit, Pierre Garcia, Alessia Sciortino, Velma T.E. Aho, Beatriz Pardo Rodriguez, Mélanie H. Thomas, Jean-Jacques Gérardy, Irati Bastero Acha, Rashi Halder, Camille Cialini, Tony Heurtaux, Irina Ostahi, Susheel B. Busi, Léa Grandmougin, Tuesday Lowndes, Yogesh Singh, Eric C. Martens, Michel Mittelbronn, Manuel Buttini & Paul Wilmes, Fiber deprivation and microbiome-borne curli shift gut bacterial populations and accelerate disease in a mouse model of Parkinson’s disease, Cell Reports, 26 September 2023.
DOI: https://doi.org/10.1016/j.celrep.2023.113071
Funding: This project received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 863664, P. Wilmes). Further support came from the Luxembourg National Research Fund (FNR) (M. Buttini, K.J. Schmit, P. Wilmes) and the Jean Think Foundation (Luxembourg) (M. Buttini).
Top image credit: Dr Kristopher Schmit