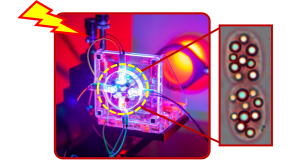
The European Research Council (ERC) will fund Prof. Anupam Sengupta’s research into how bacteria interact with and control light, and possible opportunities for new imaging technologies. The grant of 2,4 million euros will support advanced microscopy and spectroscopic analysis to examine both laboratory-grown bacteria and those from natural ecosystems.
How bacteria handle light: more than meets the eye
Light usually behaves predictably in the natural world, travelling through air, water, or glass. But a recent discovery by Prof. Anupam Sengupta, Head of the Physics of Living Matter Group, hints at something far more surprising: certain bacteria may be shaping and guiding light within their own bodies. This ability comes from tiny granular structures within the cells, previously thought to serve only as energy-storing organelles which get activated when nutrients are limiting. Understanding this opens a new perspective on how bacteria interact with light and could inspire new imaging technologies, for example, methods which could allow scientists to observe living tissues or tumour cells without powerful lasers.
To explore this new dimension of light-matter interactions, Prof. Anupam Sengupta has been awarded the prestigious ERC Consolidator Grant (ERC-CoG) worth €2.4 million by the European Research Council, for his project MicroPAS: Microbial Photonics Across Scales.
“When we observed how these globules interact with light, it became clear that they might be doing much more than simply storing nutrients,” says Prof. Sengupta. “It raises the exciting possibility that cells are biophysically shaping light in their interior, a phenomenon we never expected to find in microbes. Light can now be captured and “trapped” within cells! Thanks to these miniature lenses, even a low power light source can allow us to illuminate what’s in there.”
Comparing bacteria from controlled and natural environments
MicroPAS will integrate advanced microscopy and spectroscopic analysis to examine both laboratory-grown bacteria and those from natural ecosystems like the Lake Cadagno in Switzerland and Lake Stechlin in Germany. Prof. Sengupta has been observing these natural aquatic ecosystems for nearly a decade. They offer unique settings where light availability governs interactions and feedback, which will ultimately influence how light-harnessing species grow and survive. The experiments, complemented by data-driven numerical modeling, will pioneer a completely new class of light-based systems inspired by biology. This framework could lead to Living Photonic Circuits, where organelles control, guide and even programme light paths within living cells.
‟ Nature often reveals its beauty and its mysteries to us, but only if we’re patient enough to observe them closely.”
Associate professor, FNR ATTRACT Fellow
When microbes become Light Engineers
Phototrophic microorganisms—species that use sunlight to drive metabolism like the purple sulfur bacteria—sit at the foundation of many aquatic food chains. Their ability to harvest light efficiently is crucial to their survival. Traditionally, researchers have studied how microorganisms interact with light mainly through photosynthesis, a well-known biochemical process by which energy from the sunlight is converted into usable chemical energy, ultimately supporting the diverse metabolic needs of a cell.
But Prof. Sengupta’s research suggests that there’s more than meets the eye. Curiously, he has detected such light manipulating organelles within non-phototrophic bacteria as well, yet these internal structures have never been explored beyond their role as energy-storing units. For instance, many bacteria dwelling in the human gut or in extreme environments like hydrothermal vents, harbour tiny globules made of elemental sulfur or carbonates. These globules may be optically active, although until now scientists believed bacteria used them only for storage, or to control their movements in the watery environments. Prof. Sengupta’s team has now challenged this long-standing wisdom. In a recent discovery, they found evidence that these globules directly shape light inside cells, influencing how the bacteria function, move or store energy internally.
Turning the question around: how growth and movement influence light inside bacteria
It is well known that light from the environment can influence how bacteria grow and behave. But much less is understood about the opposite: how the physical changes inside these bacteria—such as in the shape, size or packing of organelles—might in turn affect the path of light that enters a cell.
“These organisms live in highly dynamic environments, not least in today’s context of rapid climatic shifts and evolving lifestyles.” Prof. Sengupta explains. “By studying them at different stages of growth and under ecologically relevant settings, we can start to understand how their physical and biological worlds intertwine. This knowledge will drive the next-generation of nature-inspired systems, offering unprecedented possibilities for bio-based photonic technologies for the future.”
Intracellular light guidance: the microbial way!
Understanding how light behaves within a single living cell could reshape light-guided applications, including high-speed communication and energy-efficient computing. Further afar, considering the essential role bacteria play in local and global energy flows, MicroPAS will uncover the role of optically active intracellular organelles in energy cycles across scales. Any new knowledge about the internal light-guiding strategies will fundamentally redefine the role of such microorganisms in a range of ecosystems where light is limiting or unevenly distributed.
‟ With MicroPAS, we have a cutting-edge opportunity to explore a dimension of microbial life that remains completely uncharted. It reminds us that even the simplest organisms can hold many surprises. As physicist Richard Feynman once said, ‘There’s plenty of room at the bottom’; an adage that has for long inspired the cross-disciplinary research we conduct in my team. This is even more relevant now, as we gear up to explore, and redefine the interfaces between physics and biology, thanks to this ERC-Consolidator Grant!”
Associate professor, FNR ATTRACT Fellow