On World Water Day, let’s talk about the “forever chemicals” that made a splash when the Forever Pollution Project published the map of nearly 23,000 contaminated sites all over Europe and again when the Pesticide Action Network Europe revealed that drinking water samples from 11 EU countries showed the widespread presence of trifluoroacetic acid (TFA). Prof. Emma Schymanski and Dr Federica Piras, researchers at the university focusing on chemicals in our environment, help us take a closer look at the facts, explore the long history of these compounds and reflect on the current situation in our water.
Chemicals with a long history
The term “forever chemicals” is used to refer to a large category of synthetic chemicals called per- or polyfluoroalkyl substances (PFAS). They are characterised by a very stable bond formed by carbon and fluorine in their structure. The connection between these two elements, one of the strongest in organic chemistry, does not break easily, hence the label “forever chemicals”.
PFAS were first documented in the late 1930s and are now widely used for their properties as water and grease repellents and their resistance to heat. They can be found in products as diverse as wall paint, waterproof fabric, mobile phone screens and cosmetics. As they resist degradation and are easily transported in the environment, the health effects of the legacy PFAS, the substances originally used by the industry, have been extensively studied. Their hazardous nature was publicised through examples such as the DuPont scandal, when a plant released perfluorooctanoic acid (PFOA) for years and contaminated local water supply in Parkersburg, West Virginia (USA), a story that inspired the movie Dark Waters. Two legacy PFAS, called PFOS and PFOA, have been linked to disorders such as cancers, premature birth and thyroid diseases, and their use has been regulated by the Stockholm Convention on Persistent Organic Pollutants since 2009 (PFOS; restriction) and 2019 (PFOA; elimination).
“Unfortunately, their regulation triggered a mechanism known as “regrettable substitution”, where industries developed new compounds to replace the legacy PFAS,” explains Prof. Emma Schymanski, head of the Environmental Cheminformatics group at the Luxembourg Centre for Systems Biomedicine (LCSB). “Over time, we have seen that some of the shorter chain PFAS that replaced PFOS and PFOA also remain in the environment and cause undesirable health effects.” Today, according to the latest definitions, it is estimated that there are over 7 million chemicals belonging to the PFAS category.
TFA, an emerging problem
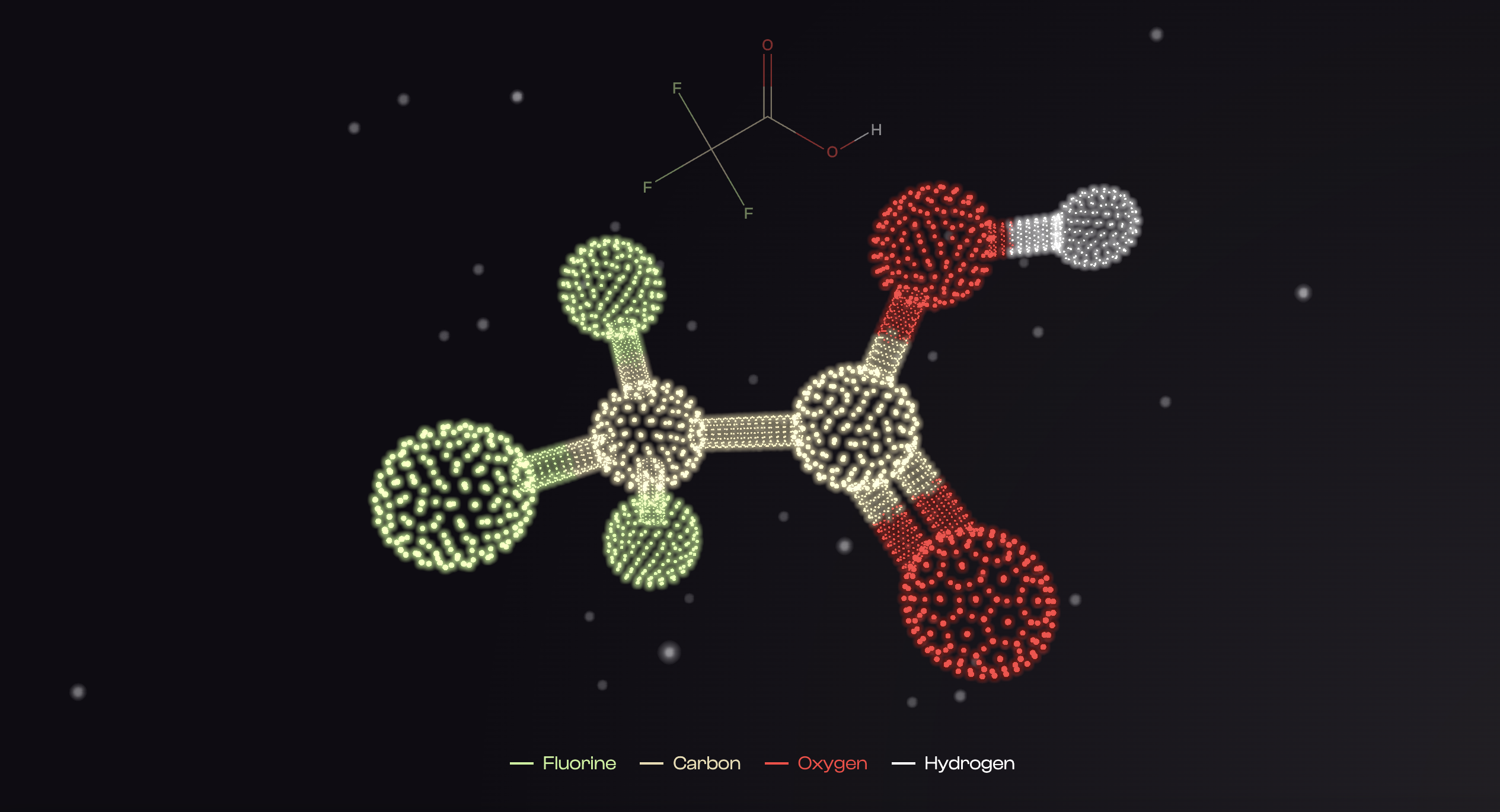
3D representation of the chemical structure of TFA (credits C2DH, 3D molecules)
Among the numerous “forever chemicals” researched over the years, one is now gaining increased attention: Trifluoroacetic acid (TFA). Manufactured for industry but also occurring as the result of the degradation of other PFAS, its concentration in the environment is increasing at an alarming rate. “There are countless potential sources of TFA, many of which are still unknown, and we are also lacking studies on its effects,” details Prof. Schymanski. “What we do know is that if this trend continues and, worse, if adverse health effects are found, then we will be faced with a massive challenge.” As a matter of fact, TFA is extremely difficult – and thus expensive – to remove, especially if there are multiple diffuse sources of TFA entering our environment. A recent investigation by French journalists estimated that cleaning up Europe’s PFAS pollution would cost between €95 billion and €2,000 billion over 20 years.
While the situation seems to call for a total ban of all PFAS, as strongly suggested by some scientists, it seems a very difficult task. “How do you implement a regulation that bans 7 million compounds?” asks Dr Federica Piras, post-doctoral researcher in the Environmental Cheminformatics group. Other options would be to control industrial waste before it enters the environment, to refine the treatment options and to keep a close eye on levels of pollutants in water sources, to see which PFAS are particularly problematic – and thus the priority for further measures. This is where research can help.
Local research to tackle the issue
“My project explores the ability of a nature-based technology, called constructed wetlands, to limit the emission of PFAS from wastewater treatment plants and aims to implement a less costly and less energy-intensive treatment compared to existing methods,” explains Dr Piras. Getting rid of PFAS entirely is extremely difficult because most of the processes tend to concentrate the chemicals rather than destroy them, ending up with residues that are even more problematic. This is where nature could provide a solution: In the right setting, different organisms like plants, bacteria and fungi, can coexist and cooperate synergically to degrade PFAS.
“Our idea is to combine ozonation with constructed wetlands, not only to abate the load of PFAS but also to cope with the removal of other contaminants of concern present in water. We want to investigate to which extent each living organisms in constructed wetlands can contribute to PFAS removal,” details Dr Piras. The goal of the LCSB researchers is to test the feasibility of such a system in rural areas of Luxembourg and to address various aspects, for example the amount of space needed for implementation or the working capacity of the wetlands. This highly interdisciplinary project, called PFAS-QUEST, is funded through the Young International Academics programme hosted by the Institute for Advanced Studies at the University of Luxembourg. It is conducted in close collaboration with Prof. Joachim Hansen and Dr Silvia Venditti, from the Urban Water Management group at the Department of Engineering of the university, and other international partners.
Additionally, Prof. Schymanski’s team is involved in analysing water all over the country to get a better overview of the situation in Luxembourg: “I understand that people are concerned but our tap water is highly controlled, even more so than mineral water, and there is a real awareness of the issue at the national level and a willingness to find solutions. This is a major motivation for our work.”
Staying informed, a key step
Despite the wide coverage, water is not the only pressing issue when it comes to exposure to chemicals. They are present everywhere in our environment, from the obvious cigarette smoke to the less apparent plastic containers we use every day or even cosmetics that directly go on our skin. “Sometimes it is useful to adjust our perception of risk. The presence of PFAS in our environment shouldn’t drive people to stop drinking water, for example, and risk dehydration in summer. Other measures may reduce exposures more effectively, such as switching from disposable cups and bottles to glass, ceramic or metal ones for your daily drinks, or avoiding using skin care products that contain PFAS,” concludes Prof. Emma Schymanski.
One way to take the right proactive steps is to find reliable sources and stay informed. There as well, the University of Luxembourg is doing its part by promoting open science. Prof. Schymanski and her team are contributing to several freely available databases on existing chemicals, their structures and their properties. Making scientific information available to all, from specialists to decision-makers and the general public, is a step in the right direction. The recently released “3D molecules” website is a new addition to the communication toolbox on chemicals. Developed by C2DH designers Daniele Guido and Kirill Mitsurov, with text provided by Prof. Schymanski, it provides accessible information about TFA but also caffeine, nicotine, DDT and bisphenol S. Check it out for your first dose of information!
—
References:
- Emma Schymanski, Jian Zhang, Paul Thiessen, Parviel Chirsir, Todor Kondic & Evan Bolton, Per- and Polyfluoroalkyl Substances (PFAS) in PubChem: 7 Million, Environmental Science & Technology, October 2023.
- Dagny Aurich, Philippe Diderich, Rick Helmus & Emma Schymanski, Non-target screening of surface water samples to identify exposome-related pollutants: a case study from Luxembourg, Environmental Sciences Europe, November 2023.
- Anjana Elapavalore, Todor Kondić, Randolph Singh, Benjamin Shoemaker, Paul Thiessen, Jian Zhang, Evan Bolton & Emma Schymanski, Adding open spectral data to MassBank and PubChem using open source tools to support non-targeted exposomics of mixtures, Environmental Science: Processes & Impacts, July 2023.
- Hiba Mohammed Taha et. al [95 authors] & Emma Schymanski, The NORMAN Suspect List Exchange: Facilitating European and worldwide collaboration on suspect screening in high resolution mass spectrometry, Environmental Sciences Europe, October 2022.
