On Rare Disease Day 2025, the Luxembourg Centre for Systems Biomedicine (LCSB) of the University of Luxembourg illuminates once again its building in blue, green, pink, and purple, joining buildings across Luxembourg and around the world. This show of solidarity, organised in partnership with ALAN, the Luxembourg association for rare diseases, pays tribute to the 300 million people diagnosed worldwide with one of the more than 6000 known rare diseases, including 30,000 in Luxembourg. Many of these diseases start in childhood, are severe and still incurable, having profound impacts on patients, families, and caregivers. Rare Disease Day highlights the urgent need for continued support and increased investment in research to develop effective treatments or even cures. The LCSB is committed to uncovering the genetic and molecular mechanisms behind rare metabolic diseases. The Enzymology and Metabolism group, led by Prof. Carole Linster, is at the forefront of this effort.
Advancing research on rare neurometabolic diseases
The group uses advanced biochemical and omics techniques to explore rare neurometabolic disorders and potential therapeutic strategies. For example, with international collaborators they discovered a rare childhood disease, called PEBEL, resulting from mutations in the NAXD or NAXE genes. These mutations disrupt the body’s metabolite repair system, which normally acts like a cellular maintenance crew, clearing away toxic by-products. When this clean-up process fails, harmful substances build up, leading to progressive brain damage and other severe symptoms seen in PEBEL.
Based on the team’s work, clinicians found that niacin (a form of vitamin B3) could help reduce some of the symptoms linked to these genetic mutations, highlighting the potential for nutritional and small molecule drug interventions to enhance rare disease outcomes. In a recent article, published in Cellular & Molecular Biology Letters, Prof. Carole Linster and her team took this idea further. They used cell cultures to test a theory originally developed from a yeast model. Their experiments confirmed that toxic metabolites build up in cells lacking the NAXD enzyme. These metabolites block the activity of another important enzyme, called 3-phosphoglycerate dehydrogenase, that plays a key role in the serine biosynthesis pathway. Without enough serine, many cellular processes are disrupted, which could help explain the symptoms seen in children with PEBEL. The researchers also discovered that this metabolic blockage becomes worse when cells are exposed to metabolic stress.
In addition, the team found a potential way to help the cells recover: Supplementation with nicotinamide riboside (NR), another form of vitamin B3, and inosine partially restored serine production and improved cell function. “While we confirmed the known beneficial effect of vitamin B3 supplementation, we found that NR specifically and other supplements, such as inosine, could have an even better effect, either as replacements for vitamin B3 or in combination with it,” explains Prof. Linster. “By gaining deeper insights into the molecular mechanisms of the disease in cell culture models, we not only enhance our understanding of the enzymatic processes involved but also pave the way for more effective, optimised treatment strategies.” These small molecules could indeed serve as promising therapeutic candidates for rare neurometabolic disorders such as PEBEL disease.
Collaborations with researchers in Australia and internal partnerships within the LCSB, including the Developmental and Cellular Biology group led by Prof. Jens Schwamborn and the Metabolomics and Lipidomics Platform, were integral to the success of the study. Future preclinical research using animal models, such as zebrafish and mice, will further investigate the therapeutic potential of these compounds.
Learning from rare diseases to tackle more widespread metabolic disorders
Building on their expertise in enzyme function discovery and metabolite repair disorders, the researchers recently applied their know-how to a gene that is more commonly mutated in the human population and strongly linked with vitamin B12 deficiency, which in turn is associated with neurodegenerative diseases, particularly in individuals with low dietary intake of the vitamin.
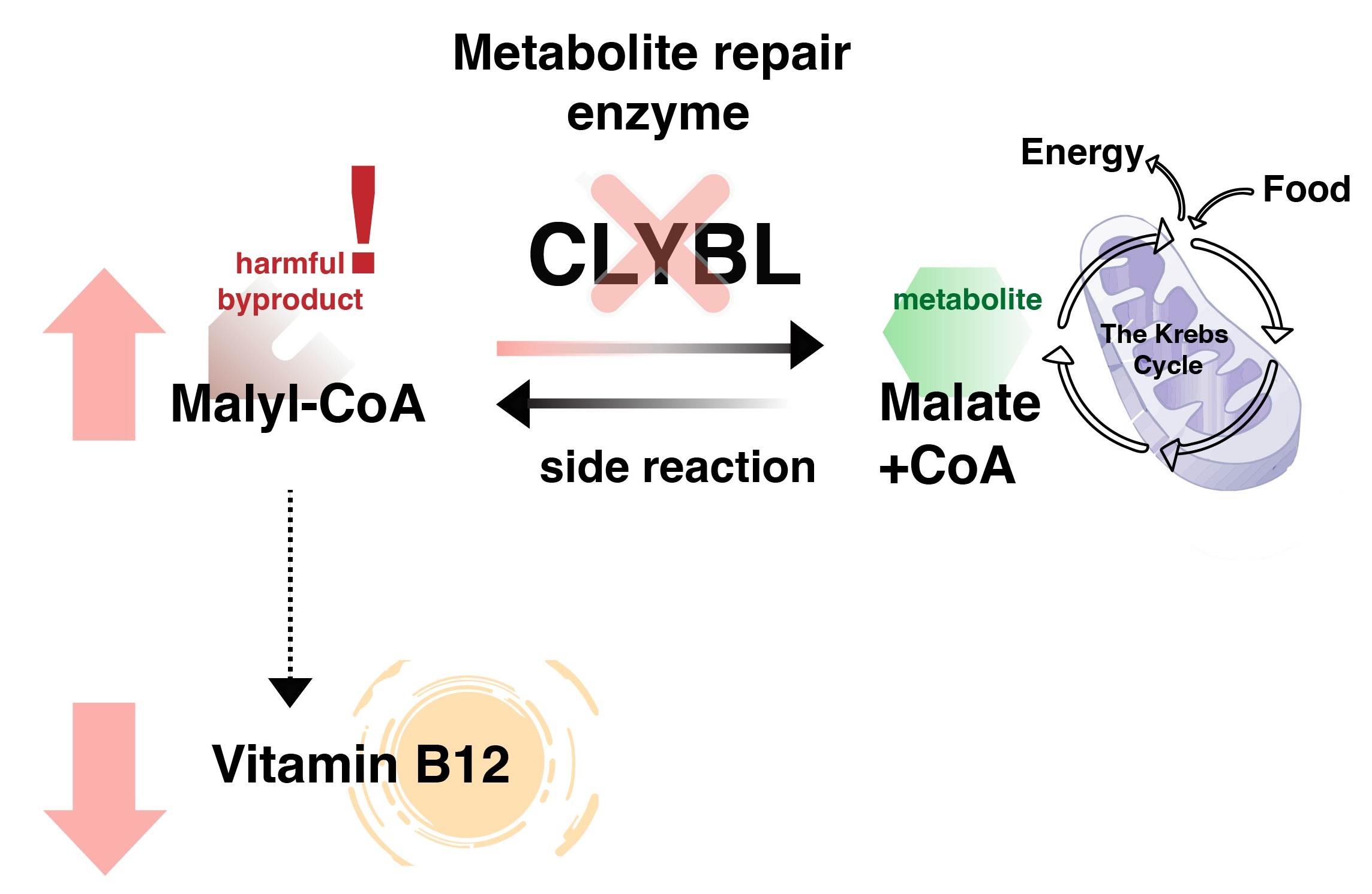
In their latest study, recently published in Nature Chemical Biology, Prof. Carole Linster and her team have discovered that this vitamin B12-linked gene encodes a new metabolite repair enzyme called CLYBL. Acting like a “clean-up crew,” CLYBL efficiently breaks down malyl-CoA to safeguard vitamin B12-dependent processes.
Relying on state-of-the-art metabolomics techniques, the scientists detected Malyl-CoA in human cells for the first time, provided evidence that it is a byproduct of the Krebs cycle, the vital process that helps to convert food into energy, and showed that it mediates the effect of CLYBL deficiency on vitamin B12 metabolism.
Interestingly, a mutation in the CLYBL gene, which causes a loss of function and impairs the enzyme’s activity, is not rare. In fact, this genetic variant appears in an unusually high percentage of the population. “While seemingly detrimental due to its link to vitamin B12 deficiency, this mutation may have provided an evolutionary advantage, explaining why it is more common in the population. It may for example help to limit the virulence of pathogenic bacteria that rely on this vitamin for their growth and survival,” underlines Prof. Linster. “Our findings challenge the notion that metabolite repair disorders are limited to rare conditions. We see here how a relatively common genetic variation can influence vitamin metabolism in a significant portion of the population.”
Moving forward, it will be interesting to investigate whether individuals deficient in CLYBL are at higher risk of developing neurological problems and whether they would benefit from vitamin B12 supplementation.
Raising awareness and engaging the community
In addition to its research, the LCSB is committed to raising awareness and supporting those affected by rare diseases. At the 2024 Researcher’s Days, a biennial science fair organised by the Luxembourg National Research Fund (FNR), the Enzymology and Metabolism group hosted an interactive workshop on rare diseases. Hundreds of visitors of all ages were able to decrypt the genetic code, experiment with enzymes, and explore how rare diseases are studied in cells and zebrafish or diagnosed in children.
Continuing the tradition started last year, when the Biotech II building was lit up for the first time in the colours of Rare Disease Day, the LCSB remains dedicated to raising awareness and supporting patients through innovative science, collaborative efforts, and outreach activities.
Its dedicated scientists are committed to studying disease mechanisms, paving the way for novel treatment approaches. To this aim, the LCSB recently established a Rare Childhood Disease Research Fund to support research on rare diseases. Join us in making a difference: Your donation can help accelerate vital research and bring hope to children and families affected by rare diseases.
—
Recent scientific publications:
- Walvekar AS, Warmoes M, Cheung D, Sikora T, Seyedkatouli N, Gomez-Giro G, Perrone S, Dengler L, Unger F, Santos BFR, Gavotto F, Dong X, Becker-Kettern J, Kwon YJ, Jäger C, Schwamborn JC, Van Bergen NJ, Christodoulou J & Linster CL. Failure to repair damaged NAD(P)H blocks de novo serine synthesis in human cells. Cellular & Molecular Biology Letters. January 2025.
- Griffith CM, Conrotte JF, Paydar P, Xie X, Heins-Marroquin U, Gavotto F, Jäger C, Ellens KW, Linster CL. CLYBL averts vitamin B12 depletion by repairing malyl-CoA. Nature Chemical Biology. March 2025.
Funding:
This research was supported by the University of Luxembourg, the Luxembourg National Research Fund (FNR), the European Union’s Horizon 2020 Research and Innovation Programme, and a donation from the Juniclair Foundation.