Alzheimer’s disease (AD), the leading cause of dementia, does not affect everyone equally: women are at higher risk, even when accounting for the fact that they generally live longer than men. They also often experience more severe symptoms than men. However, the molecular mechanisms behind these differences were not clear. A new study has now uncovered sex-specific molecular changes in Alzheimer’s in different cell types of the brain, suggesting new targets for personalised therapeutic approaches. The study, led by Prof. Enrico Glaab of the Luxembourg Centre for Systems Biomedicine (LCSB) at the University of Luxembourg, has recently been published in Alzheimer’s & Dementia.
Zooming down to single cell level
Using advanced single-cell analysis, the research team studied more than 2.3 million brain cells, including both neurons and different types of glia cells, from people with Alzheimer’s disease and healthy controls who had donated their brains to research after death. They were particularly interested in the pre-frontal cortex, a key area responsible for decision making and memory retrieval. The researchers measured the gene expression levels of thousands of genes in individual cells, a process also referred to as single-cell transcriptomics. They then compared the changes between patients and healthy controls but also between the two sexes in the affected and non-affected groups to look for sex-dependent changes. This innovative approach allowed them to analyse in unprecedented detail gene activity patterns and cellular processes across multiple types of brain cell that play a role in the disease.
Their findings revealed distinct molecular signatures in men and women with Alzheimer’s. In male patients, astrocytes, a subtype of glial cells responsible for metabolic, structural, homeostatic, and neuroprotective functions, showed significant changes in pathways associated with cell death. In contrast, female patients showed alterations in pathways involved in cellular communication and growth regulation. “By focusing on individual cells, we can investigate sex-dependent disease mechanisms and subtle differences between cell types of the brain that might otherwise remain hidden,” explains Mohamed Soudy, PhD student in the Biomedical Data Science group at the LCSB and first author of the study.
Sex differences in cellular communication
A key feature of Alzheimer’s disease is a breakdown in how brain cells communicate with each other, leading to neuronal death and widespread loss of brain function. The researchers found indications that some of these communication disruptions manifest differently between the sexes. Male AD brains showed pronounced changes in cell death signalling, while female brains showed altered calcium signalling, a key process in brain function and memory. “These sex-specific differences and the complex interplay of cellular processes provide a clearer picture of how Alzheimer’s manifests distinctly in men and women,” says postdoctoral researcher Dr Sophie LeBars, who co-authored the study.
Proposing different drug targets for men and women
To look for potential drug targets, the researchers used computational models that depicted how molecular regulators interact with each other and with other substances in the cell to govern gene expression levels. Through the systematic analysis of these cell type-specific gene regulatory networks and by simulating potential drug effects, key regulatory molecules were identified as candidate drug targets that could revert sex-dependent pathological changes. In male patients, RPTOR, a molecule involved in cell survival and metabolism, was identified as a potential mediator of sex-specific changes in Alzheimer’s disease. In female patients, the researchers identified LRP1 as a key regulatory molecule. This protein plays an important role in clearing toxic proteins such as amyloid-beta from the brain. LRP1’s position as a central mediator in female-specific gene networks in Alzheimer’s suggests it could merit further investigation as a target for sex-specific therapies.
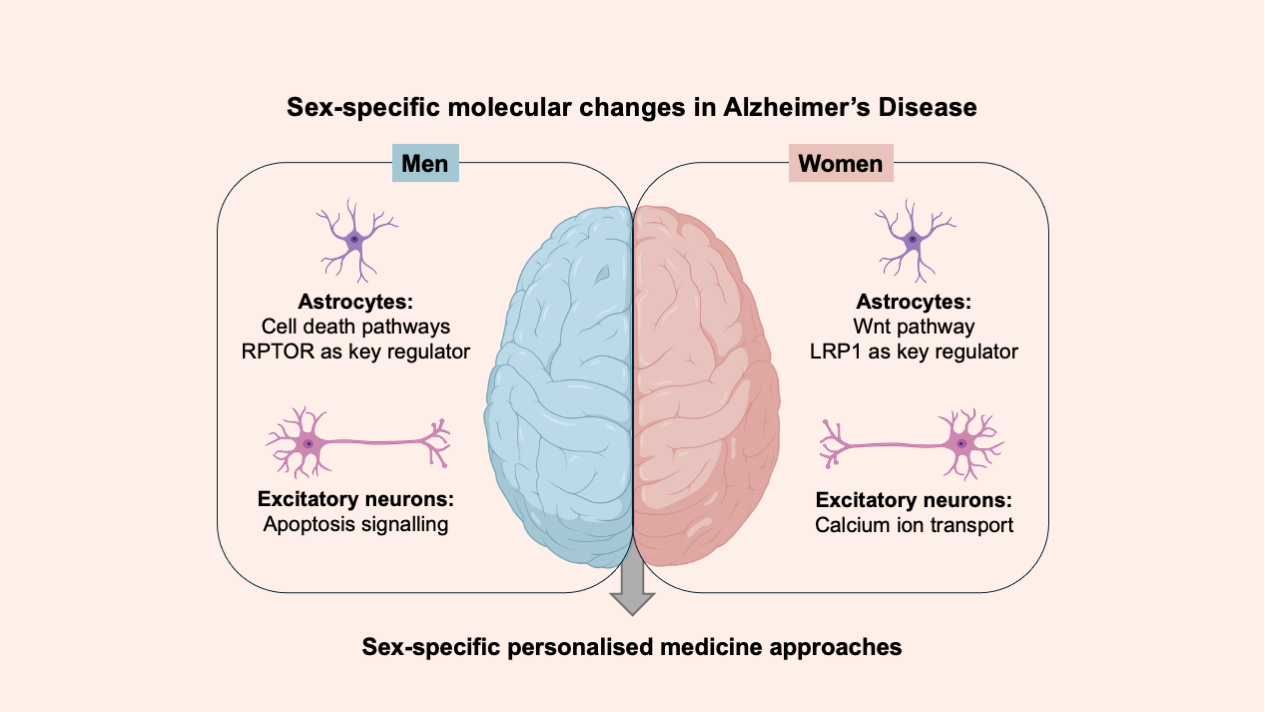
“These findings highlight the benefits of considering sex as an important biological variable in Alzheimer’s research,” says Prof. Enrico Glaab, principal investigator of the Biomedical Data Science group and senior author of the study. “Tailoring treatments to such molecular differences could improve therapeutic efficacy and pave the way for more personalised medicine.” An approach urgently needed as the global burden of Alzheimer’s disease continues to rise.
Scientific publication: Mohamed Soudy, Sophie Le Bars and Enrico Glaab, Sex-dependent molecular landscape of Alzheimer’s disease revealed by large-scale single-cell transcriptomics, Alzheimer’s & Dementia, December 2024.
